The New Patent Law in New Zealand
Pharmaceutical Dose Forms – Design Patent and Trademark Intellectual Property
12 years ago
By Steven J. Hultquist, Principal
© Hultquist, PLLC, 2013. All rights reserved.
Although the principal focus is on composition and use patents in securing intellectual property rights in pharmaceutical dose forms – the physical form of a medicine or therapeutic agent that is administered to a patient for treatment or prevention of an adverse physiological condition – the value added by design patents and trademark protection should not be overlooked.
When the dose form is a tableted form, the shape, coloration, and overall conformation of the tablet may be susceptible to design patent protection. As an example, Pfizer, Inc.’s U.S. Design Patent 434,136 contains the following drawings among the drawing figures defining the scope of its proprietary right under this patent
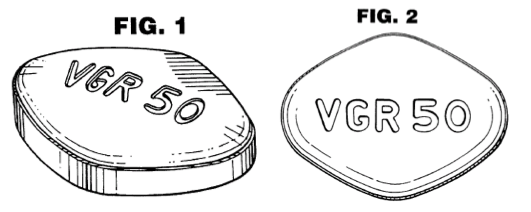
in which the horizontal line shading in the upper right corner of the tablet shown in FIG. 1 represents the color blue and extends to cover all surfaces of the claimed design.
Tablets can therefore be considered topological articles, for which color schemes, as well as features of the structure such as lands, grooves, depressions, protrusions, circumferential bands, and the like, can be the substantive basis of design patent protection as well as ornamental features that are susceptible to trademark protection. As an example, see Merck’s U.S. Trademark Registration 3,510,220 for the following color scheme for nutritional supplement tablets
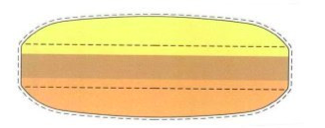
in which the description of the trademark is set out in the registration as follows:
The color(s) light yellow, light brown and ochre is/are claimed as a feature of the mark. The mark consists solely of the color combination of light yellow, light brown and ochre as applied to a drug capsule with three color layers. The shape of the drug capsule is indicated in dotted lines to show that it is not part of the mark and only serves to show positioning of the features of the mark, and, a transparent outer layer is shown representing a protective coating which is not a feature of the mark. The layer below the protective coating appears in the color light yellow and represents vitamins; the middle layer appears in the color light brown and represents minerals and trace elements; and the layer below the light brown layer appears in the color ochre and represents probiotic cultures.
Another example is provided by Sanofi’s U.S. Trademark Registration 3,737,092 for pharmaceutical tablets having a peanut shape
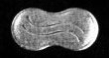
for the following pharmaceutical products specified in the registration
Pharmaceutical preparations, namely, preparations for the prevention and treatment of cardiovascular diseases; pharmaceutical preparations, namely, preparations for the prevention and treatment of cancer; pharmaceutical preparations, namely, preparations for the prevention and treatment of thrombosis; pharmaceutical preparations, namely, preparations for disease prevention and treatment in the field of internal medicine; pharmaceutical preparations, namely, preparations for the prevention and treatment of diseases of the central nervous system; pharmaceutical preparations, namely, preparations for the prevention and treatment of respiratory diseases
in which the trademark is described in the registration in the following terms
The mark consists of a three-dimensional oval shape whose long sides pinch inwards, similar to a peanut. Carved into one side of the shape are two parallel wavy lines.
As is evident from the foregoing, visual aesthetic aspects of dose forms afford a path to enhancing the proprietary position that can secured for these pharmaceutical products.
Concerning the applicable criteria for determining whether these trademark and design patent protections are available, trademarks must have sufficient distinctiveness to establish brand identity and be free from likelihood of confusion in the marketplace, and design patents require that the design be ornamental in character, and satisfy novelty and nonobviousness requirements in relation to the applicable “prior art.”
Among all of these criteria, the nonobviousness requirement for design patent protection is frequently the most difficult to assess. Recently, the Court of Appeals for the Federal Circuit has clarified this requirement in High Point Design LLC v. Buyers Direct, Inc., No. 2012-1455 (September 11, 2013), identifying the applicable standard for determining obviousness of a design patent as being “ordinary designer” standard, viz., whether the claimed design would have been obvious to a “designer of ordinary skill” who designs articles of the type involved.
The existence of the above-discussed design patent and trademark forms of intellectual property rights therefore raise the issue of potential infringement when a new pharmaceutical dose form is being developed, as to which it is necessary to locate and assess existing, adversely-held design patents and trademarks that may impact the new dose form, and create liability exposure.
This so-called “freedom to operate” assessment in the case of trademarks looks at the issue of likelihood of confusion in the marketplace for the new dose form color/conformation trademark in the context of existing applicable color/conformation branding, to determine whether those in the marketplace would be likely to experience confusion regarding the source of products when the new “visually branded” dose form is considered against existing products in that marketplace.
The freedom to operate assessment in the case of design patents looks at infringement risk under a basic test established by the U.S. Supreme Court in 1871, known as the “ordinary observer test” since it provides that infringement exists if “in the eye of an ordinary observer, giving such attention is a purchaser usually gives, two designs are substantially the same”. See Gorham Co. v. White (U.S. 1871).
More recently, the Court of Appeals for the Federal Circuit has held that this ordinary observer test should be conducted in the context of applicable prior art. See Egyptian Goddess v Swisa, 543 F. 3d 665 (Fed. Cir. 2008) (en banc) (“relying on the ordinary observer test, conducted in light of the prior art”).
It therefore is necessary in applying the ordinary observer test to rigorously determine the strength, scope, and validity of the design patent against which the new pharmaceutical product is considered.
The pharmaceutical market is becoming progressively more competitive, as generic drug-makers worldwide turn increasingly to developing proprietary active pharmaceutical ingredients (APIs) and API formulations to bolster margins over those of “me-too” products, and as brand houses in the brand-versus-generic and brand-versus-brand battles seek to extend the reach of their proprietary positions in all possible ways against all competitors.
In this milieu, color/conformation trademarks and design patents offer the potential for increased intellectual property protection of new pharmaceutical dose forms, beyond a historical approach focused solely on securing composition and use patents.
Steven J. Hultquist, Principal
Hultquist, PLLC
Chapel Hill, NC
October 19, 2013
